Managing aging and frailty in the urologic cancer population
Increasing numbers of older patients with cancer necessitates adoption of an age-friendly approach to cancer care.
Tullika Garg, MD, MPH, FACS

The worldwide population is aging, a demographic shift fueled by the 73 million baby boomers who were born between 1946 and 1964. Cancer incidence rises over the course of an individual’s lifetime and peaks by the eighth decade, making it an important condition of aging. By 2030, all baby boomers will be older adults and more than two-thirds of all new cancers will be diagnosed in individuals 65 years and older.1 In particular, the population of the “oldest old” (aged ≥ 85 years) is expected to triple, and cancer is the second most common cause of death in this group.2 Urologic cancers are of special concern for the aging population with projected increases in the incidence of prostate, bladder, and kidney cancer in older adults of 71%, 68%, and 67%, respectively. By 2030, approximately one-quarter of all new cancer diagnoses in individuals over age 85 years will be prostate or bladder cancer.2 Urologists are the front line in the diagnosis and treatment of urologic cancers. As a specialty, we must be prepared for the unique and rewarding challenges of caring for an increasingly older and medically complex patient population in the coming years.
Our ultimate goal as surgeons caring for older adults with cancer is to right-size our approach by avoiding undertreatment or overtreatment. Undertreatment is defined as providing less than standard-of-care treatment to a fit older adult who may benefit from more intensive treatment. In contrast, overtreatment is providing intensive treatment to a vulnerable older adult who may benefit from a more tailored approach.3 Unfortunately, our traditional oncologic fitness measures (eg, Karnofsky and ECOG performance status) are not accurate for assessing an older adult’s tolerability for cancer treatment.4,5 Older adults are a heterogeneous population, and traditional measures do not incorporate frailty and geriatric conditions that better determine an older adult’s ability to tolerate medical or surgical cancer treatment. Frailty is defined as a loss of physiologic reserve that results in reduced ability to bounce back from stressors, such as surgery or chemotherapy.6 Geriatric conditions are different from comorbidities or single-organ chronic conditions because they result from impairments in multiple organ systems. Some examples of geriatric conditions include cognitive impairment, multimorbidity, functional status, and falls, among others (Figure). Frailty and geriatric conditions are often linked, as individuals may become frail with the gradual accumulation of geriatric conditions.
From geriatric oncology data, we have learned that cancer treatment exacerbates geriatric conditions or leads to the development of new conditions. For example, cancer diagnosis and treatment in older adults is associated with cancer-related cognitive decline, falls, and new coexisting chronic conditions.7-9 Although our current understanding of how major cancer surgery impacts geriatric outcomes (eg, functional decline) is more limited, some data are emerging. The GOSAFE study is 1 of the only large, international longitudinal studies of older adults undergoing cancer surgery.10,11 Of 942 older adults undergoing major cancer surgery (median age, 78 years) at 26 centers, 20% had baseline cognitive impairment, one-third had weight loss or nutritional deficiency, and 36% to 68% had positive indicators for frailty, depending on the tool used. Recently, 6-month quality of life data were published from GOSAFE showing that older adults who screened as frail on the Flemish Triage Risk Screening Tool had higher odds of worsening quality of life following major cancer surgery (odds ratio [OR], 1.58; 95% CI, 1.13-2.21). Longitudinal functional outcomes are still pending. One limitation of the study is that it enrolled only 12 older adults undergoing urologic cancer surgery (nephrectomy, prostatectomy, and radical cystectomy), and more data specific to geriatric outcomes following urologic cancer surgery are badly needed.
Given the heterogeneity of older adults, chronological age alone is also not adequate to determine an older adult’s tolerance for cancer treatment. Current guidelines from the American Society of Clinical Oncology, the National Comprehensive Cancer Network, the American College of Surgeons, and the American Urological Association recommend screening for frailty and assessing baseline geriatric conditions as a standard part of pretreatment evaluation for older adults.12-14The most comprehensive tool is the geriatric assessment (GA), which is a systematic evaluation of geriatric conditions and domains.15 Three recent landmark studies (GAP70+, GAIN, and INTEGERATE) have shown that GAs and GA-driven interventions improve aging-focused conversations around treatment decisions and grade 3-5 toxicities in older adults with cancer undergoing systemic therapies.16-19
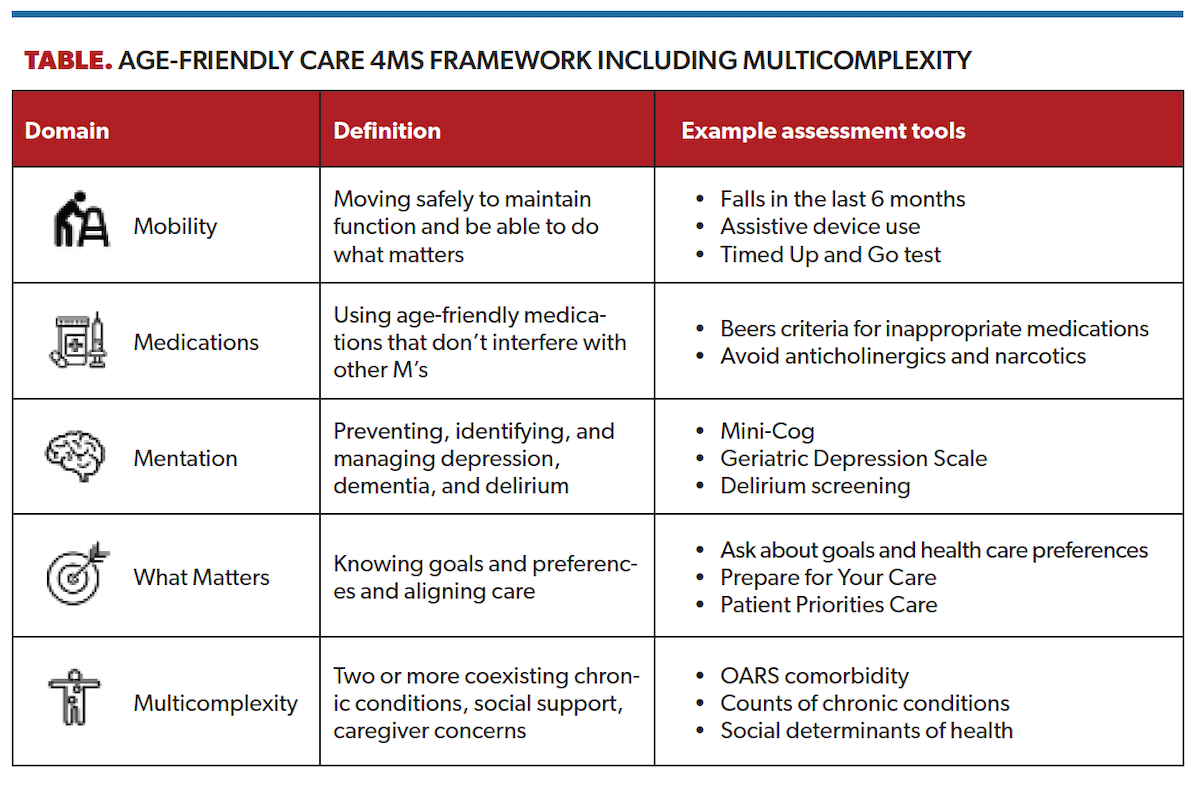
Ideally, GA would be implemented into our surgical urology clinics to arm patients, families, and urologists with data to facilitate risk stratification, decision-making, and supportive care interventions. However, as a practical matter, even survey-based GAs like the Cancer and Aging Research Group tool are difficult and time-consuming to implement into busy surgical clinics. As an alternative, frailty screening tools such as the Geriatric-8 and the Vulnerable Elders Survey-13 are shorter and can be used to identify older adults who may benefit from a referral to a geriatrician for a comprehensive GA. Another option is to focus on a core set of validated tools guided by the Institute for Healthcare Improvement’s Age-Friendly Health Systems 4Ms framework (Table).20 The 4Ms domains are mobility, medications ,mentation, and what matters. Within geriatrics, a fifth M is often added for multicomplexity, which is relevant to older adults with urologic malignancies given high rates of coexisting chronic conditions (multimorbidity) and associated care complexity.21,22
“What matters” is one of the most critical 4Ms domains, as older adults with serious illnesses such as cancer value nontraditional outcomes in addition to oncologic ones.23 For example, an older adult with cancer may decline a treatment that would lead to cognitive impairment or loss of independence resulting in a nursing home admission. Asking about and documenting an older adult’s goals and health care preferences is a key part of providing age-friendly care and underpins patient-centered decision-making in older adults with cancer.
Shifting worldwide demographics means all urologists will be caring for an ever-greater proportion of older patients with cancer with complex needs in the very near future. Older adults with cancer are unique because they manage geriatric conditions and frailty, and have a desire to maximize nontraditional outcomes in addition to traditional oncologic ones. As a specialty, we must quickly equip ourselves with the tools and knowledge to provide personalized, right-sized, and age-friendly cancer care that aligns with each individual’s goals and health care preferences.
References
1. Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7-33. doi:10.3322/caac.21654
2. DeSantis CE, Miller KD, Dale W, et al. Cancer statistics for adults aged 85 years and older, 2019. CA Cancer J Clin. 2019;69(6):452-467. doi:10.3322/caac.21577
3. DuMontier C, Loh KP, Bain PA, et al. Defining undertreatment and overtreatment in older adults with cancer: a scoping literature review. J Clin Oncol. 2020;38(22):2558-2569. doi:10.1200/JCO.19.02809
4. Hurria A, Mohile S, Gajra A, et al. Validation of a prediction tool for chemotherapy toxicity in older adults with cancer. J Clin Oncol. 2016;34(20):2366-2371. doi:10.1200/JCO.2015.65.4327
5. Bergerot CD, Philip EJ, Bergerot PG, et al. Discrepancies between genitourinary cancer patients’ and clinicians’ characterization of the Eastern Cooperative Oncology Group performance status. Cancer. 2021;127(3):354-358. doi:10.1002/cncr.33238
6. Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146-156. doi:10.1093/gerona/56.3.m146
7. Leach CR, Weaver KE, Aziz NM, et al. The complex health profile of long-term cancer survivors: prevalence and predictors of comorbid conditions. J Cancer Surviv. 2015;9(2):239-251. doi:10.1007/s11764-014-0403-1
8. Ahles TA, Root JC. Cognitive effects of cancer and cancer treatments. Annu Rev Clin Psychol. 2018;14:425-451. doi:10.1146/annurev-clinpsy-050817-084903
9. Sattar S, Haase K, Kuster S, et al. Falls in older adults with cancer: an updated systematic review of prevalence, injurious falls, and impact on cancer treatment. Support Care Cancer. 2021;29(1):21-33. doi:10.1007/s00520-020-05619-2
10. Montroni I, Rostoft S, Spinelli A, et al. GOSAFE - Geriatric Oncology Surgical Assessment and Functional rEcovery after Surgery: early analysis on 977 patients. J Geriatr Oncol. 2020;11(2):244-255. doi:10.1016/j.jgo.2019.06.017
11. Montroni I, Ugolini G, Saur NM, et al. Quality of life in older adults after major cancer surgery: The GOSAFE International Study. J Natl Cancer Inst. 2022;114(7):969-978. doi:10.1093/jnci/djac071
12. Mohile SG, Dale W, Somerfield MR, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO Guideline for Geriatric Oncology. J Clin Oncol. 2018;36(22):2326-2347. doi:10.1200/JCO.2018.78.8687
13. NCCN. Clinical Practice Guidelines in Oncology: Older adult oncology, version 2.2022. Accessed February 11, 2023. https://www.nccn.org/professionals/physician_gls/pdf/senior.pdf
14. ACS NSQIP/AGS Best Practice Guidelines: Optimal preoperative assessment of the geriatric surgical patient. American College of Surgeons and American Geriatrics Society; 2012:1–52. Accessed February 11, 2023. https://www.facs.org/-/media/files/quality-programs/nsqip/acsnsqipagsgeriatric2012guidelines.ashx
15. Rostoft S, O’Donovan A, Soubeyran P, Alibhai SMH, Hamaker ME. Geriatric assessment and management in cancer. J Clin Oncol. 2021;39(19):2058-2067. doi:10.1200/JCO.21.00089
16. Mohile SG, Mohamed MR, Xu H, et al. Evaluation of geriatric assessment and management on the toxic effects of cancer treatment (GAP70+): a cluster-randomised study. Lancet. 2021;398(10314):1894-1904. doi:10.1016/S0140-6736(21)01789-X
17. Kleckner AS, Wells M, Kehoe LA, et al. Using geriatric assessment to guide conversations regarding comorbidities among older patients with advanced cancer. JCO Oncol Pract. 2022;18(1):e9-e19. doi:10.1200/OP.21.00196
18. Soo WK, King MT, Pope A, Parente P, Darzins P, Davis ID. Integrated Geriatric Assessment and Treatment Effectiveness (INTEGERATE) in older people with cancer starting systemic anticancer treatment in Australia: a multicentre, open-label, randomised controlled trial. Lancet Healthy Longev. 2022;3(9):e617-e627. doi:10.1016/S2666-7568(22)00169-6
19. Li D, Sun C-L, Kim H, et al. Geriatric Assessment–Driven Intervention (GAIN) on chemotherapy-related toxic effects in older adults with cancer. JAMA Oncol. 2021;7(11):e214158. doi:10.1001/jamaoncol.2021.4158
20. Mate KS, Berman A, Laderman M, et al. Creating age-friendly health systems - a vision for better care of older adults. Healthc (Amst). 2018;6(1):4-6. doi:10.1016/j.hjdsi.2017.05.005
21. Garg T, Young AJ, Kost KA, et al. Burden of multiple chronic conditions among patients with urological cancer. J Urol. 2018;199(2):543-550. doi:10.1016/j.juro.2017.08.005
22. Tinetti M, Huang A, Molnar F. The geriatrics 5M’s: a new way of communicating what we do. J Am Geriatr Soc. 2017;65(9):2115. doi:10.1111/jgs.14979
23. Fried TR, Bradley EH, Towle VR, Allore H. Understanding the treatment preferences of seriously ill patients. N Engl J Med. 2002;346(14):1061-1066. doi:10.1056/NEJMsa012528
